Aicone Biochip
World’s first fully quantitative, COVID-19 Antibody Test – Full titres of all Ig classes/subclasses, IgG, IgA, IgM; IgG1-4
Introduction
Aicone Biochip is a multi-antigen, multi-domain, fully quantitative serology COVID-19 test.
Aicone Biochip™ enables the determination of the target epitopes, titres and Ig class/sub-class (IgG, IgA, IgM; IgG1-4) of antibodies produced across all stages of COVID-19 infection, from initial exposure, disease development, post-recovery or post-vaccination. These results will provide critical insights on whether individuals have high titres of potentially neutralising antibodies against SARS-CoV-2 that may protect against reinfection or whether booster vaccinations are required.
Until recently there was no COVID-19 antibody test available to provide a quantitative result. That has changed. The Aicone Biochip test is based on the differential ratio between Ig class/sub-classes across the 4 SARS-CoV-2 antigens to give an estimation of an individual’s stage of infection and immunity. This is unmatched by any other test.
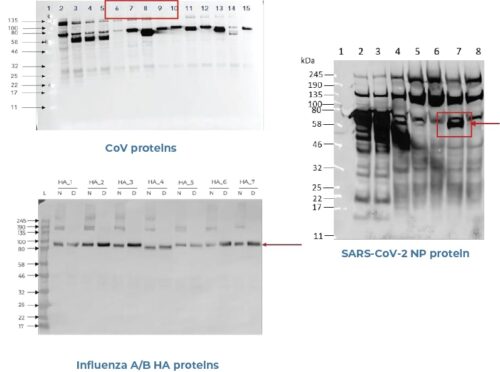
Aicone Biochip is a lab-based biochip test that utilises protein folding technology ensuring that viral antigens are correctly folded, preserving all conformational and linear antibody binding sites. Aicone-Protein Array which is utilized by major pharma and research entities to develop vaccines and therapeutics, ensures that the test identifies more true positives and less false negative.
Cross-reactivity between immunogenic regions of SARS-CoV-2 and other coronaviruses may lead to an overestimation of sero-prevalence in a given population. Aicone Biochip is designed to reduce cross-reactivity by targeting multiple SARS-CoV-2 specific domains, resulting in fewer false positives, and a more accurate determination of sero-prevalence.
The Aicone Biochip product range is biochip-based. They all incorporate multiple additional viral antigens. They are designed for research and clinical vaccination trials. See the Product Description for full details.
Content can be customised based on individualised requirements for vaccine clinical trials or sero-prevalence research studies.
KEY APPLICATIONS
Population antibody profiling for sero-prevalence research
The target epitope of antibodies produced following infection exhibit a significant level of diversity across a population. Aicone Biochip provides a more accurate determination of sero-prevalence by targeting over 10 different domains of SARS-CoV-2 proteins.
Determine the protective quality and quantity of antibodies produced
Some individuals produce antibodies that target locations which are neutralising (protective), whereas others produce non-neutralising antibodies. Aicone Biochip can be used to identify individuals who have high titres of potentially neutralising antibodies against SARS-CoV-2.
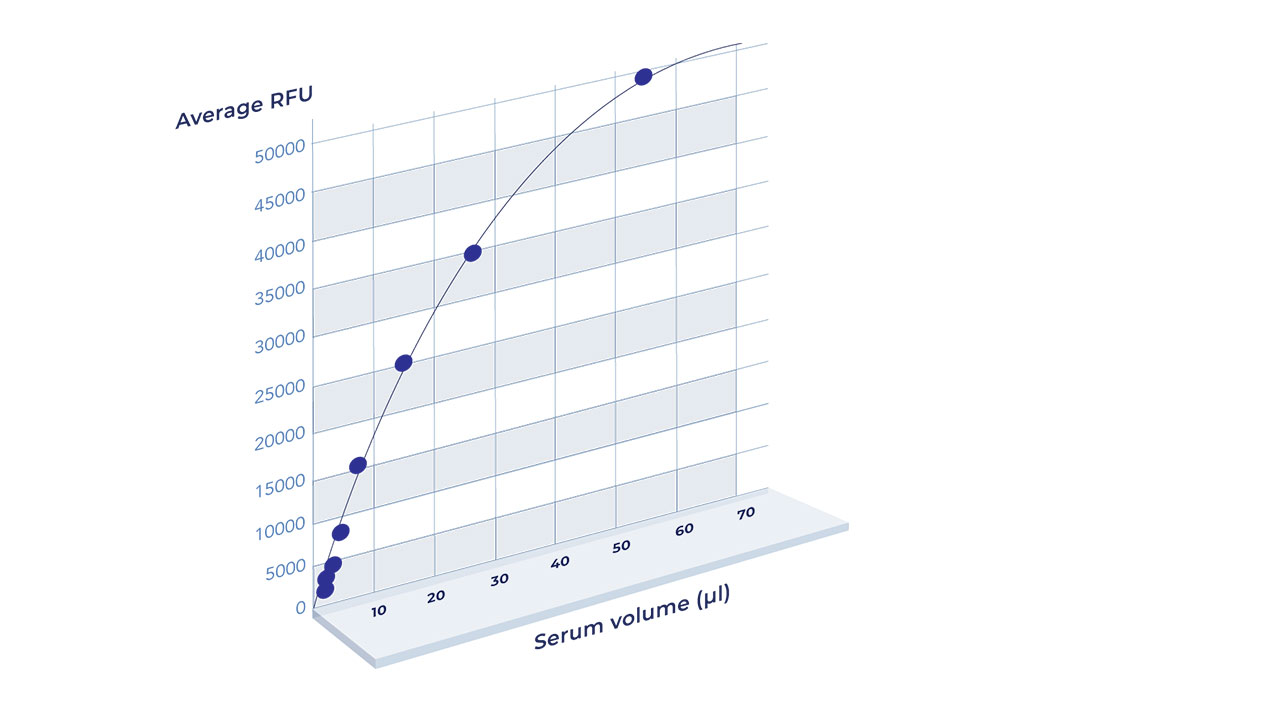
Enable quantitative assessment of antigen-specific response by individuals in vaccine trials
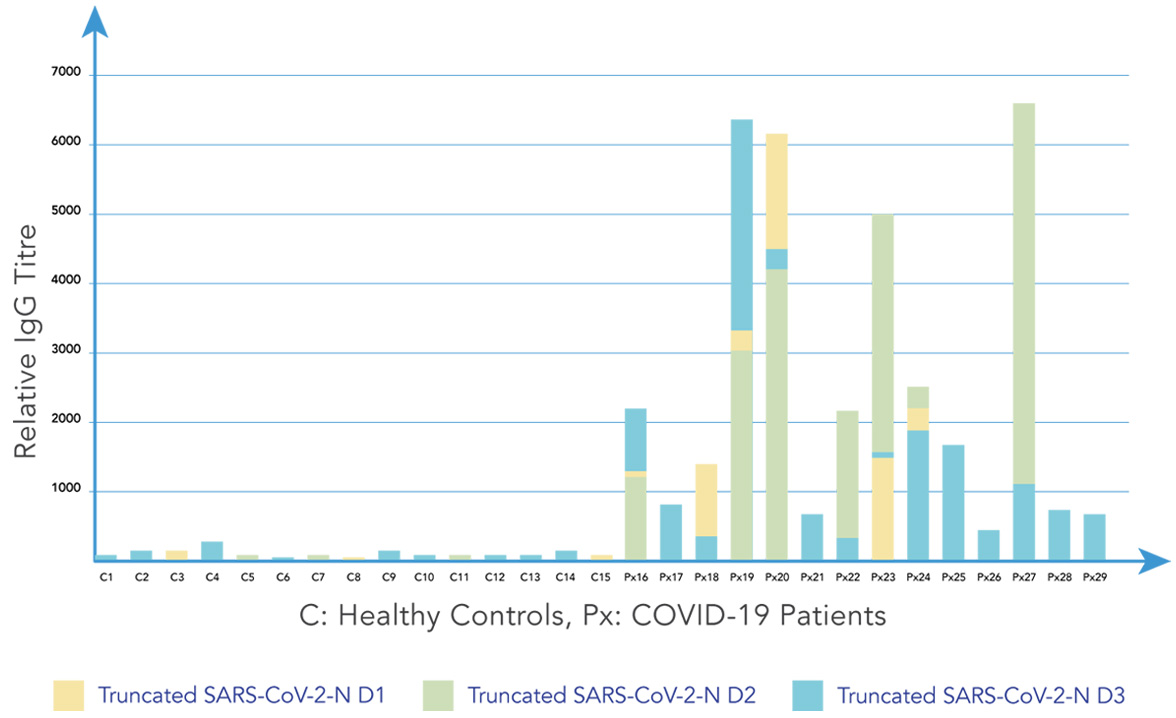
As shown below, data from Aicone Biochip tests enable 3 major conclusions to be inferred:
1. There is a wide variation in antibody titres across SARS-CoV-2 infected individuals
2. There is a high degree of variation in patient-specific antibody responses to the different domains of SARS-CoV-2 proteins
3. Wider coverage of target epitopes leads to increased sensitivity and specificity
AICONE BIOCHIP PRODUCT RANGE
Product Description
39508H and 39508V have 24 arrays per slide, 39508R has 8 arrays per slide.
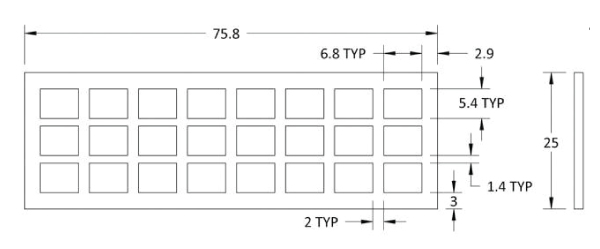
Product Specification
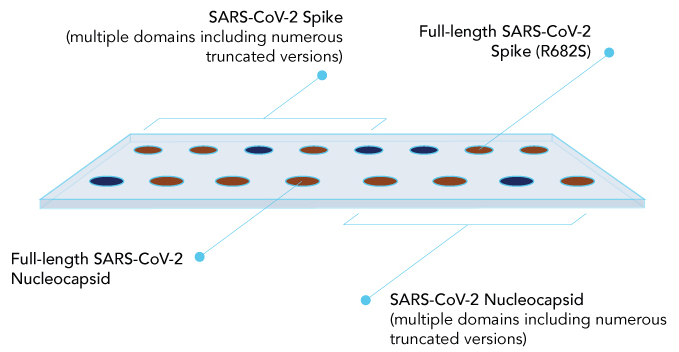
Biochip Layout
Control Probes
- Positive control
-
- Cy3-BSA
- Cy5-BSA
- IgA (positive control for total immunoglobulins detection)
- IgG (positive control for IgG and total immunoglobulins detection)
- IgM (positive control for IgM and total immunoglobulins detection)
- Negative control
-
- IgA (negative control for IgG and IgM detection)
- IgG (negative control for IgM detection)
- IgM (negative control for IgG detection)
- Negative control probe for total immunoglobulins detection
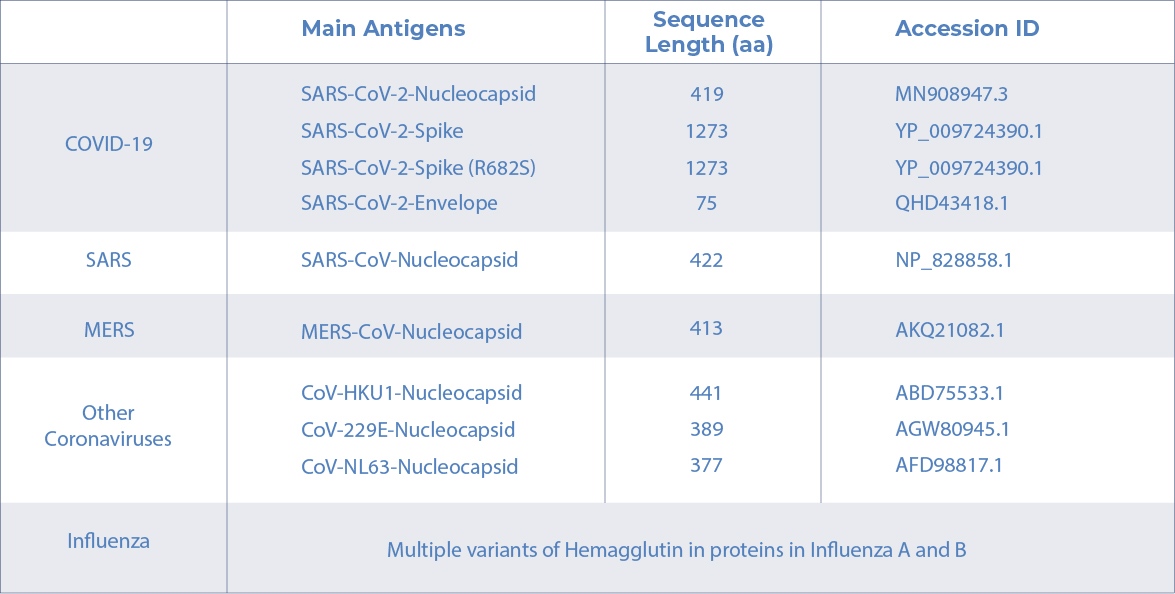
AICONE BIOCHIP ANTIGENS
ADVANTAGES OF USING AICONE-PROTEIN ARRAY
Aicone-Protein Array facilitates measures of antibodies at unrivalled sensitivity and specificity, making it a vastly superior to other antibody platforms. Because all the proteins on Aicone-Protein Array protein arrays are correctly folded, conformational as well as non-conformational epitopes are available for binding to antibodies. Since 90% of antibody binding sites are conformational in nature, if the protein is not full length or not correctly folded, other protein arrays actually lead to the loss of binding to these conformational epitopes. Using Aicone-Protein Array protein arrays ensures the binding of antibodies to not just linear, non-conformational epitopes but also to discontinuous conformational epitopes.
The main advantage of the Aicone-Protein Array based array is that the immobilised protein is folded in its native conformation, due to the BCCP fusion and the absence of physical protein purification which may affect epitope exposure for recognition.
*90% of antibodies bind to conformational epitopes, references:
- Barlow, D. J., Edwards, M. S., & Thornton, J. M. (1986). Continuous and discontinuous protein antigenic determinants. Nature, 322(6081), 747–748;
- Van Regenmortel, M. H. V. (2006, May). Immunoinformatics may lead to a reappraisal of the nature of B cell epitopes and of the feasibility of synthetic peptide vaccines. Journal of Molecular Recognition, 19(3), 183–187.
TUMOUR NECROSIS FACTOR (TNF) α – 1 antigen
Interferons – Antinuclear antibodies – Tumour Necrosis Factor
ACCURACY DUE TO AICONE-PROTEIN ARRAY
AICONE BIOCHIP Leverages AICONE-PROTEIN ARRAY World’s Only Correctly Folded Protein Technology
- Since most antibodies are thought to recognise discontinuous epitopes on their target proteins, it’s critical that antigens used in serological assays or presented to B-cells for antibody-based vaccine development are in a natively-folded form.
- The use of Aicone-Protein Array technology for the production of the SARS-CoV-2 antigens will ensure these antigens are correctly folded, preserving all conformational antibody binding sites.
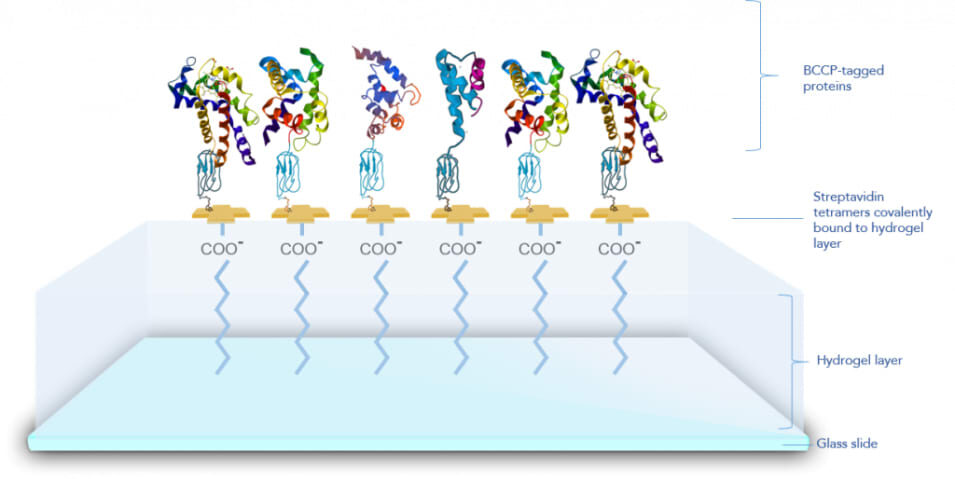
Surface Chemistry of Aicone-Protein Array
- BCCP-tagged, biotinylated Aicone-Protein Array proteins are immobilised onto a customised streptavidin-coated hydrogel surface.
- Aicone-Protein Array proteins retain their folded structure and function as if they are present in an aqueous environment.
- Proteins behave as if they are in free solution.
COMPETITIVE DYNAMICS AND TECHNOLOGY USP
Exceptional Consistency
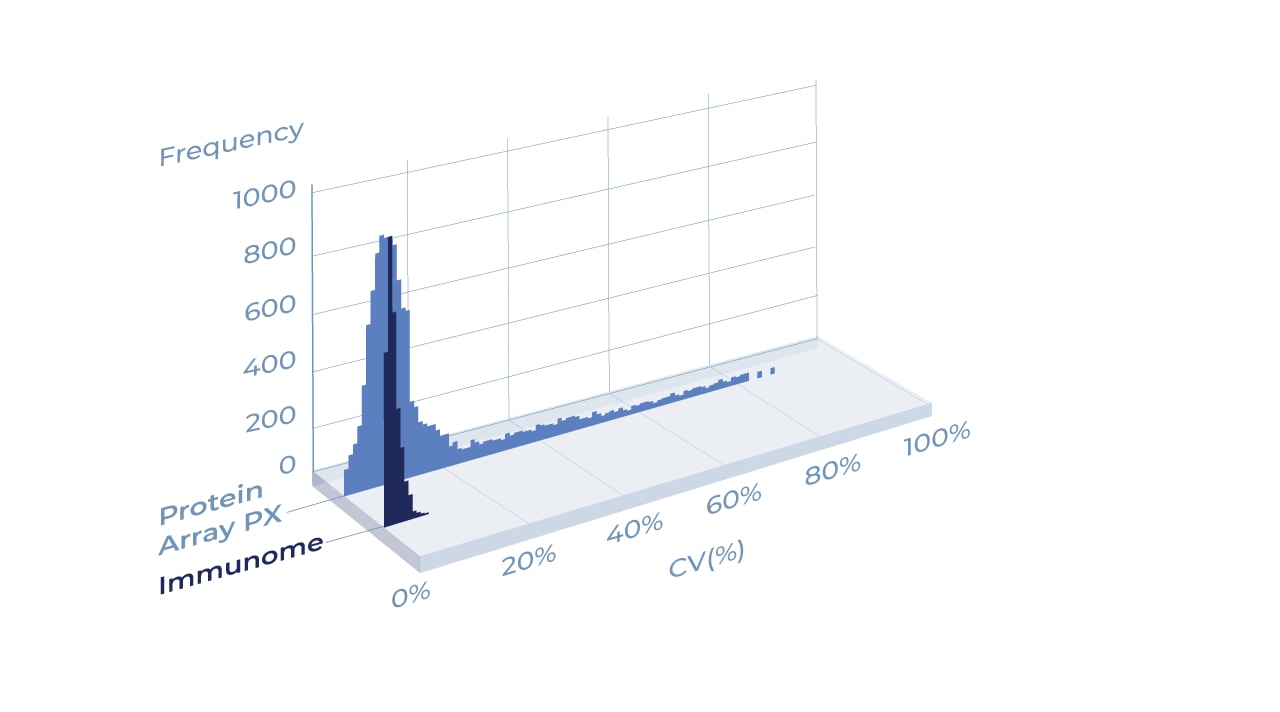
Protein arrays made with the Aicone-Protein Array technology exhibit exceptional consistency as measured by the coefficient of variation percentage (CV%).
The graph shows the CV% of intra-protein replicates run on a Aicone-Protein Array based protein array (Immunome) compared to a non-Aicone-Protein Array protein array (PX). The mean CV% for Immunome was 7.2% whereas the CV% for protein array PX was 37%.
Very high Specificity
The correct folding of proteins on Aicone-Protein Array protein arrays ensures that both continuous and discontinuous epitopes are preserved, hence all antibody binding sites are available. This leads to Aicone-Protein Array arrays having very high specificity, and by extension, a high signal-to-noise ratio.
Highly Reproducible
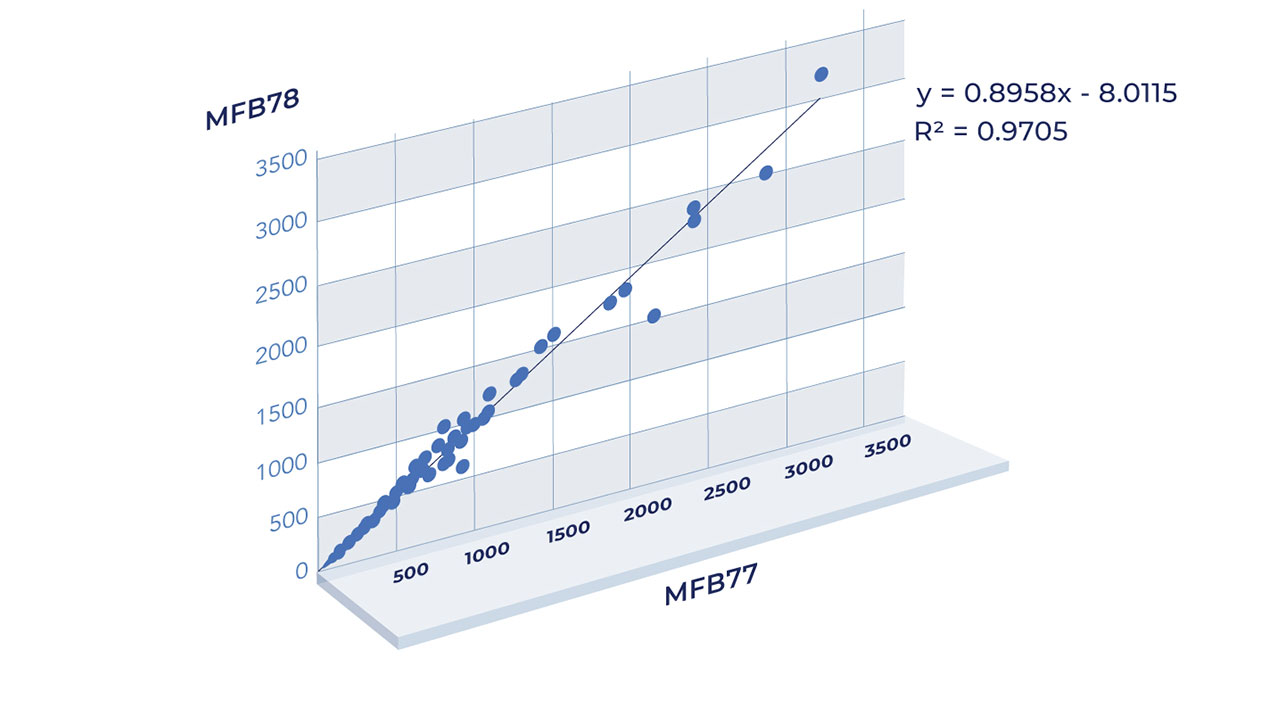
Aicone-Protein Array based protein arrays use multiple positive and negative controls to measure reproducibility within, and across, studies and batches.
The graph shows an almost perfect Pearson correlation of above 0.97 when comparing auto-antibody levels across 6,524 protein data points in two different batches.