Aicone Biochip
Introduction of ACE2 binding assay
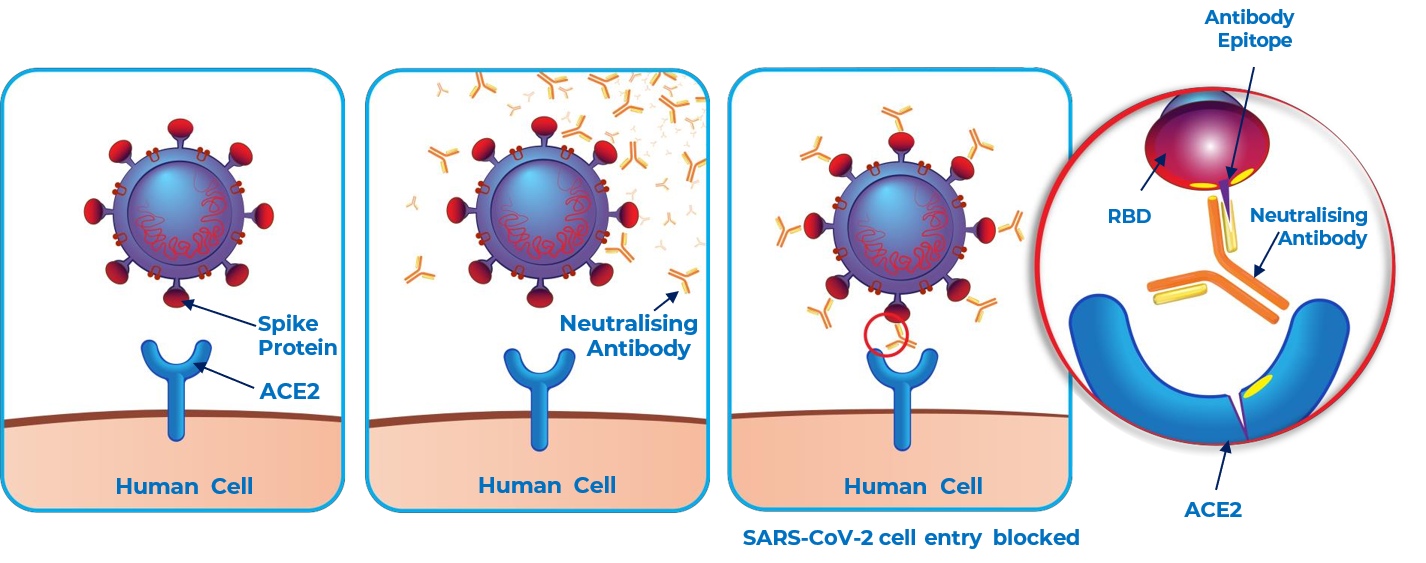
- Measuring ACE2 binding on Aicone Biochip can predict the neutralisation activity of anti-S antibody in any given sample
- Higher IgG on full-length S means higher neutralising activity
- Observations of neutralising activity measured on Aicone Biochip is in concordance with neutralising activity observed via pseudovirus neutralisation assay
- Measuring neutralising antibody on Aicone Biochip via ACE2 Assay is less laborious. Only 2 additional components needed, ACE2 protein – Cy5 conjugated and co-factor ZnCl2
Aicone Biochip – COVID+ Test introduction
Aicone Biochip is the only test in the world that not only provides information on whether you have neutralising antibodies post-vaccination and post-infection but can also tell you if you are more likely to develop severe COVID-19 symptoms as Aicone Biochip contains SARS-CoV-2 Nucleocapsid, SARS-CoV-2 Spike, Interferons (-α, -γ, -ω), Antinuclear Antibodies and Tumour Necrosis Factor α.
BCCP:
compact, inert, folding marker
Biotin is attached ONLY
to correctly folded proteins
Misfolded proteins misfold BCCP, leading to loss of its bioinylation activity
Only correctly folded proteins
are attached
Aicone Biochip is a fully quantitative, highly accurate COVID-19 test
| Sample Type | Human Serum / Plasma |
|---|---|
| Number of Arrays | 24 arrays per slide (24 samples per slide) |
| Assay Type | Single-colour fluorescently-labelled IgG antibody assay Dual-colour fluorescently-labelled IgG and ACE2 |
| System Compatibility | Any open-format microarray scanner (Agilent, InnoScan, Genepix and Tecan scanner) |
Aicone Biochip answers some of the most important questions post COVID-19 vaccination and infection
Aicone Biochip can confirm that an individual has neutralising antibodies and has generated a sufficiently strong immune response to the virus to confer protection.
Aicone Biochip can confirm the status of ongoing protection and evaluate whether a booster vaccine is required.
Aicone Biochip
as a risk predictor
Determine if an individual is more likely to develop severe symptoms and help healthcare authorities provide more targeted treatments.
Aicone Biochip has higher sensitivity than other serology tests
Comparison of Aicone Biochip arrays with single-antigen antibody tests.
- Patients selected for the study were either symptomatic or asymptomatic at the time of RT-PCR testing, with samples collected between 3 to 4 weeks after testing.
- Samples screened on Aicone Biochip array.
- Independent experiments were conducted on the same convalescent COVID-19 patients on three other major leading single-antigen antibody tests.
Aicone Biochip is able to call more true positives than antibody test, R
Aicone Biochip is able to determine titre and position of antibodies
Data from Aicone Biochip tests enable 3 major conclusions to be inferred:
- There is a wide variation in antibody titres across SARS-CoV-2 infected individuals
- There is a high degree of variation in patient-specific antibody responses to the different domains of SARS-CoV-2 proteins
- Wider coverage of target epitopes leads to increased sensitivity and specificity
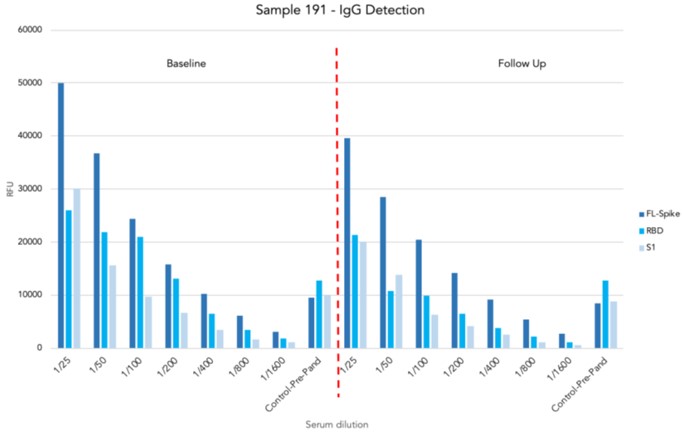
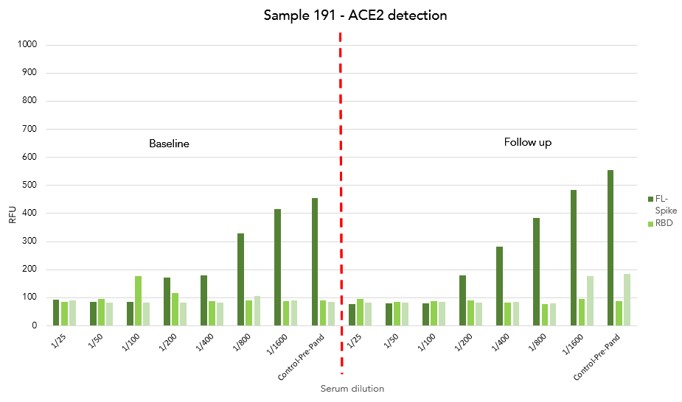
Optimisation linearity assays
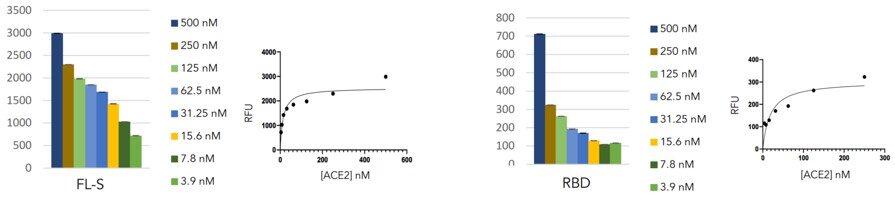
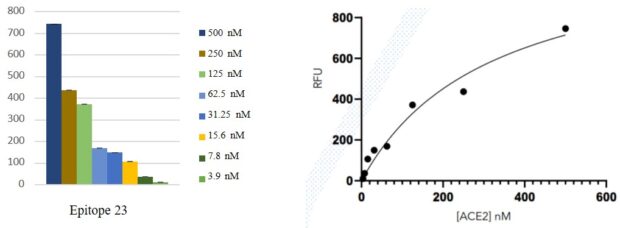
- A robust linearity trend was observed in both IgG and ACE2 detection as the serum diluted
- At the highest serum concentration (1/25), the ACE2 signal is significantly reduced close to background signal (RFU <100)
Samples with high anti-S activity showed a significant inhibition of ACE2 binding against all structural elements of SARS-CoV-2 Spike protein.
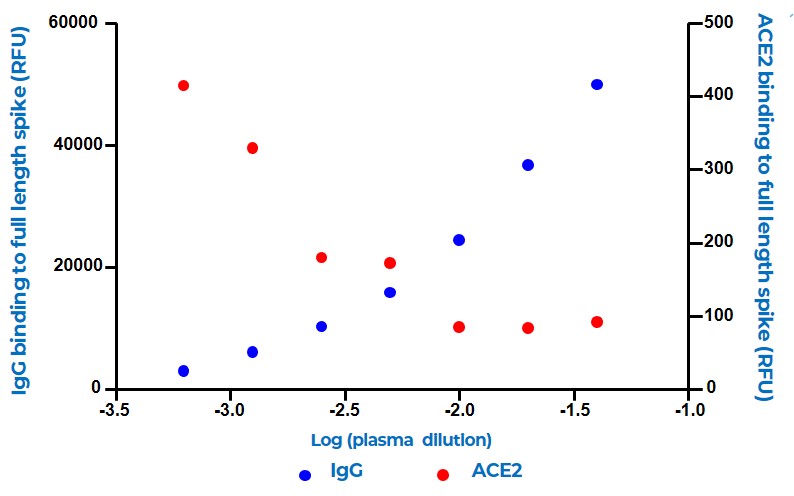
This shows that increasing levels of patient serum lead to blocking of interaction between ACE2 and the full-length Spike protein
An inverse correlation is observed between IgG titre and ACE2 binding towards full–length S protein, suggesting that inhibition of ACE2 is achieved in samples with high anti-S (FL) activity.
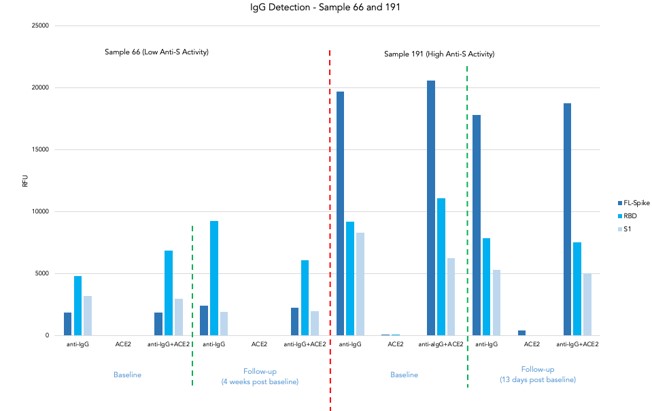
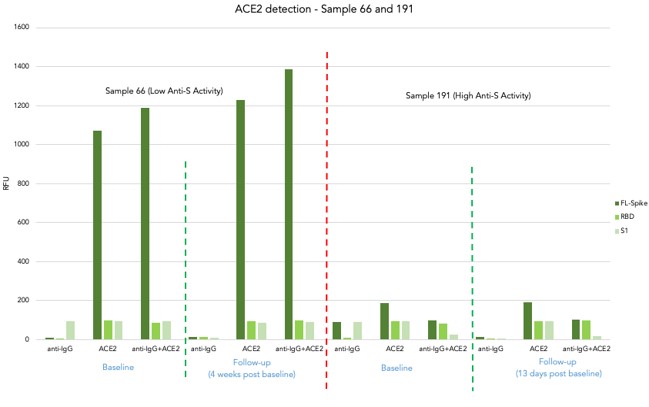
ACE2 assay on Samples 66 and 191
- 4 samples from 2 participants with known anti-S activity (Low-Sample 66 and High-Sample 191) at baseline and follow-up blood collection
- There is no cross-reactivity in IgG detection and ACE2 detection
- Samples with high IgG titre against all structural elements of S protein (Full-length, RBD and S) showed significantly low ACE2 activity in relation to samples with low IgG titre
Sample with high ab titre showed a significant inhibition of ACE2 binding towards all structural elements of SARS-CoV-2 spike protein.
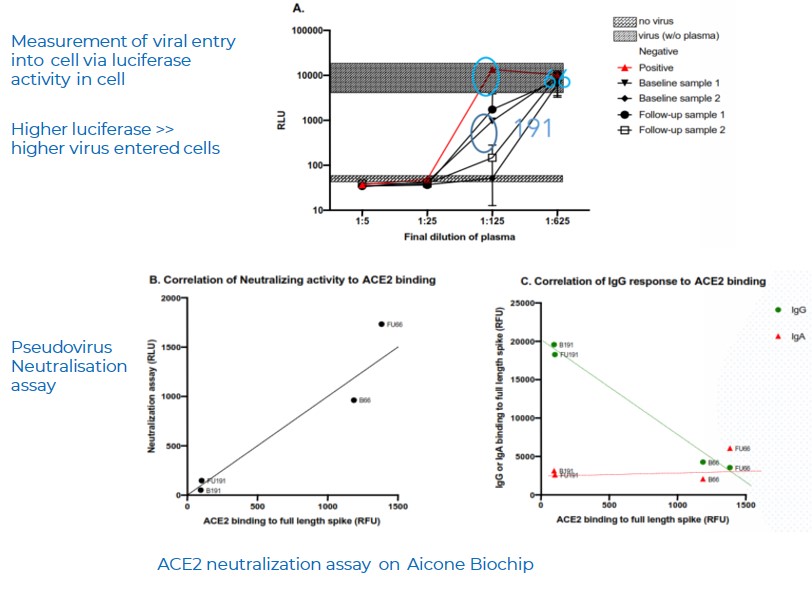
Pseudovirus neutralisation assay match ACE2 binding assay on Aicone Biochip
A.
- Blue-Known negative samples ; Red- Known positive samples
- Pseudovirus neutralisation assay confirms the presence of neutralising ab in BOTH sample 66 (sample 1) and 191 (sample 2)
- Luciferase activity is LOW in sample with HIGH IgG titre (Sample 191), indicating less viral entry into cells. Significant inhibition by anti-S antibody in sample 191.
B.
-
There was a strong correlation between neutralising activity, as measured by the pseudo-virus assay, and ACE2 binding, as measured via protein microarray assays (r = 0.9559, p-value = 0.0441*). Protein microarrays also showed that ACE2 binding correlated inversely with IgG binding (r = -0.9950, p-value = 0.005**).
C.
-
Correlation as described in (B) was not observed in IgA, suggesting that IgG is responsible for the neutralising activity, not IgA.
Conclusion of ACE2 binding assay
- Measuring ACE2 binding on Aicone Biochip can predict the neutralisation activity of anti-S antibody in any given sample
- Higher IgG on full-length S means higher neutralising activity
- Observations of neutralising activity measured on Aicone Biochip is in concordance with neutralising activity observed via pseudovirus neutralisation assay
- Measuring neutralising antibody on Aicone Biochip via ACE2 Assay is less laborious. Only 2 additional components needed, ACE2 protein – Cy5 conjugated and co-factor ZnCl2